How can Ready for Brexit help you?
We can help you redesign your business model and survive Brexit.
Our consulting team have personal experience of operating before the Single Market and Customs Union began.
The Use of Payday Loans During Brexit
Many company have reported needing to use payday loans because of Brexit. We would recommend Wage Day Advance if you do you need to use a payday lender. They can approve loans within an hour and cash paid out the same day.
We can help with the key issues:
2021 Key Brexit Dates
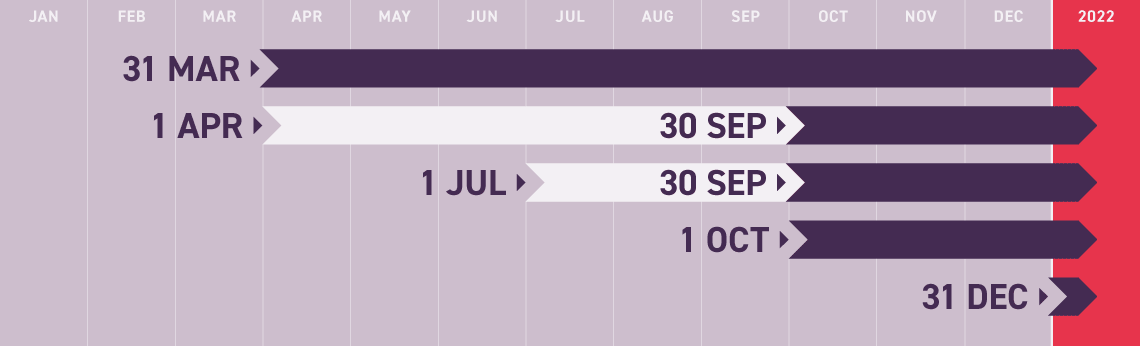
31 MAR
(Unresolved)
NOW 2022
Memorandum of Understanding for cooperation on financial services due
30 SEP
WAS 1 APR
NOW 2022
Grace period for GB-NI agri-food safety paperwork ends
30 SEP
WAS 1 JUL
NOW 2022
Grace period for GB-NI trade of chilled meat products ends
1 OCT
UNRESOLVED
NOW 2022
UK-EU border controls start to be progressively introduced
31 DEC
UNRESOLVED
NOW 2022
EU regulations on flow of GB-NI medicines enter into force